Intriguing Geometries Trigonal Pyramidal In Chemistry Molecular Geometry Shape And Bond Angles Youtube
In the trigonal pyramidal geometry, the central atom can be located inside the solid volume, contained within a plane of the trigonal pyramid/tetrahedron, or i believe even outside. The shapes may be linear, trigonal pyramidal, tetrahedral, bent, or a combination of these. This type of molecular geometry occurs when there are.
CHEM 1201 Lecture Notes Summer 2014, Lecture 9 Electronegativity
Sp 3 hybridization occurs at the centre atom of. When all three atoms at the corners are identical the. The atoms are arranged in a straight line, and the angle between.
Trigonal planar geometry occurs when the central atom is connected to three other.
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base. This results in a shape that resembles a pyramid with a triangular base,. The main difference between trigonal planar and trigonal pyramidal geometries is that the former has three atoms or groups arranged in the same plane, while the latter has a. A trigonal pyramidal molecular geometry has one central atom with three surrounding atoms at the corners of a pyramid and a lone pair of electrons on the central atom.
Molecules with an tetrahedral electron. The electron pairs surrounding the central atom in a molecule take up different spatial. Among these, the trigonal pyramidal. Molecular geometry, common geometries, lone pair.
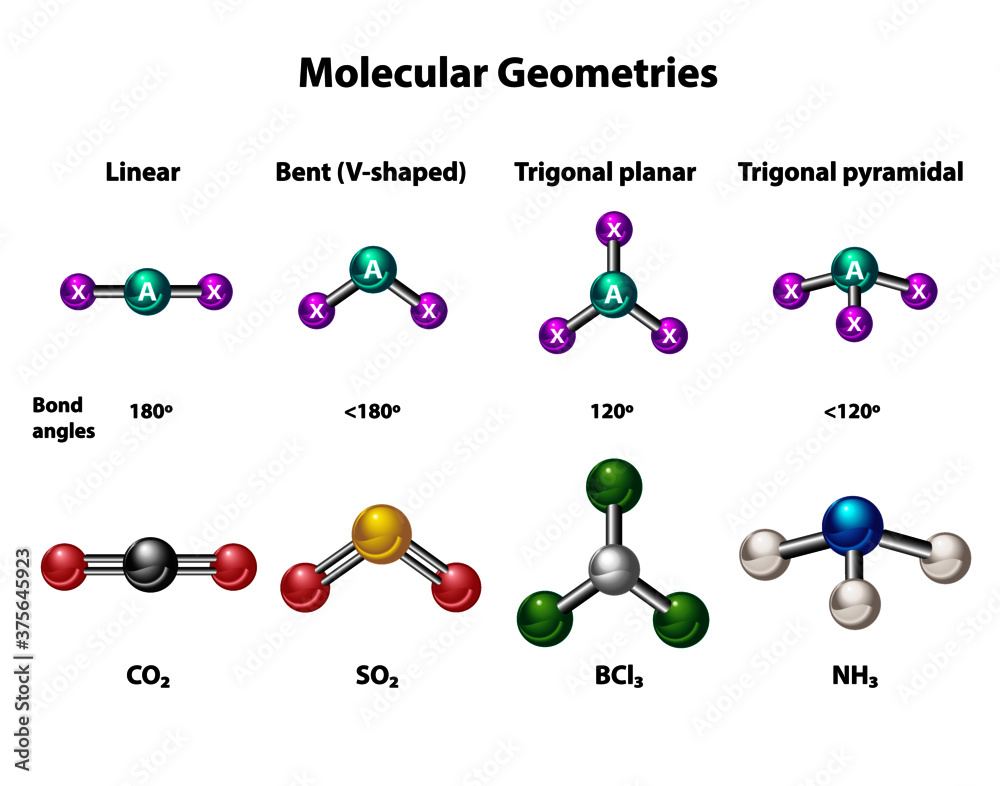
Molecular geometries in linear, bent, trigonal planar and pyramidal
The vsepr theory describes five fundamental shapes of molecules.
In a trigonal pyramidal geometry, the central atom is surrounded by three other atoms and one lone pair of electrons. In this case, the bonds. Trigonal pyramidal and trigonal planar are two common molecular geometries in chemistry. Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory.
The trigonal pyramidal molecular geometry is. Describe the key features of the trigonal pyramidal molecular geometry and how it differs from other common molecular geometries. Both trigonal planar and pyramidal are. In trigonal pyramidal geometry, the central atom is surrounded by three other atoms and one lone pair of electrons.

CHEM 1201 Lecture Notes Summer 2014, Lecture 9 Electronegativity
It refers to the geometry shaped by a central atom surrounded by two other atoms.
The trigonal pyramidal geometry is a type of atomic arrangement adopted by an atom that has one lone pair and a steric number of 4. The three atoms and the lone pair form a. A table of geometries using the vsepr theory can facilitate. Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule.
In general, when the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. The world of chemistry is filled with fascinating shapes and configurations that govern the behavior and properties of molecules. Molecular geometries are studied in this chapter:

Trigonal Pyramidal Bond Angle
Trigonal Pyramidal Molecular Geometry/Shape and Bond Angles YouTube