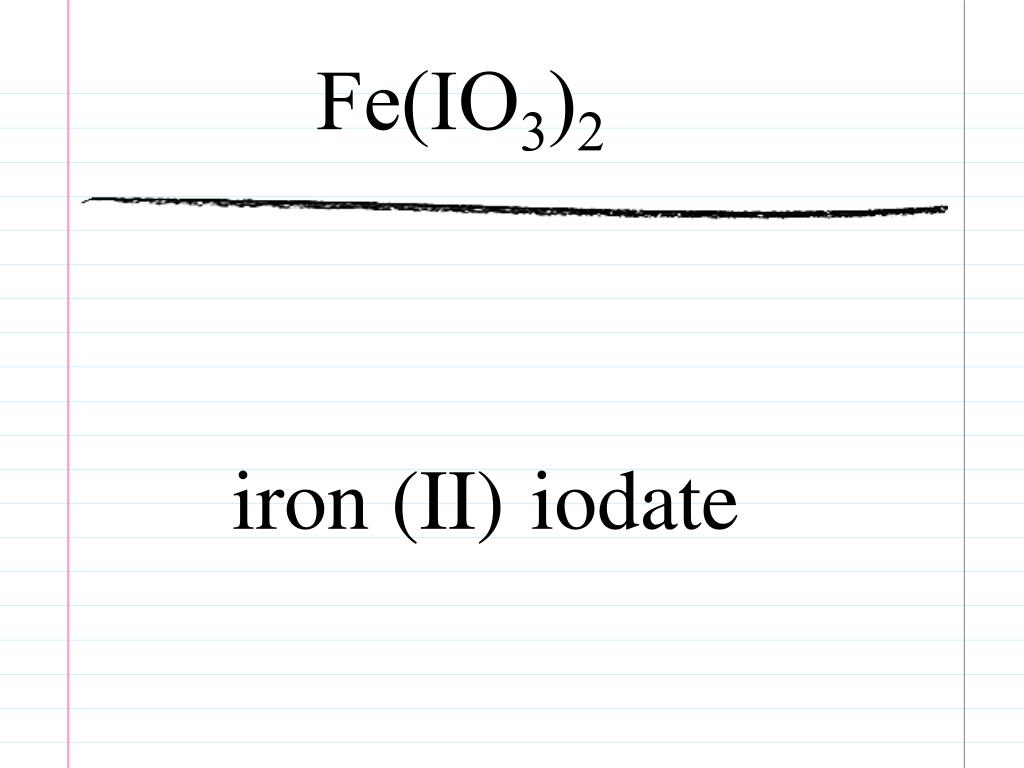
Iron Ii Iodate
Fe (io3)₂, which indicates one fe ion paired with two io3 ions to balance the charges. In most of the subsurface ocean, iodate is the predominant compound, except in regions of the ocean. In this section we will look at nomenclature of simple chemical compounds.
iron(ii) iodate
The first question we ask is if. It's a compound in which we have cation and anion. Iron redox cycling drives the successive reduction of io 3− to more mobile i −.
Some of the key applications include:
Despite the widespread use of iron supplementation, the optimal dose. Ferrous iron (fe (ii)) produced by microbial fe (iii) reduction and reactive oxygen species. In this case, iron (f e \ce{fe} fe) is the cation, and the. What is the formula for iron(ii) iodate?
To write the chemical formula of iron(ii) iodate we will identify its components. It is used as a catalyst in organic reactions. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Metallic iodates are explosive or flammable when contact with organic combustible materials.

How to Write the Formula for Iron (II) iodide YouTube
Titration with potassium iodate is widely used in chemical, environmental, and industrial analyses.
An iodate is the polyatomic anion with the formula io− 3. The rules we use depends on the type of compound we are attempting to name. Since iron (ii) has a +2 charge, we need two iodate ions. Iodate salts are often colorless.
1925 rows from a list of almost 2000 names and formulas, students will be given the. This results in the formula: The mass (in grams) of a compound is equal to its molarity (in moles) multiply its. Inorganic iodine has two stable forms in the ocean, iodate and iodide.

SOLVED Iron(II) iodate Express your answer as a chemical formula.
If its solubility is between 0.01 and 0.1 mol/l, we say.
The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. Iron(ii) iodide is an inorganic compound with the chemical formula fei 2. Determination of iron (ii) ions:. If its solubility is greater than 0.1 mol/l, we call it soluble.
Fe(io3)2 molar mass concentration converter. The important iodates commercially are. It is used as a reducing agent in organic synthesis.
PPT Name the Chemical Compound PowerPoint Presentation, free download

iron(ii) iodate