Is No2- Polar No2 Lew Structure And Molecular Geometry Youtube
Industrially, no2 is an intermediate in the synthesis. Is no2⁺ polar or nonpolar? In a polar bond, one atom is positively charged and the other is.
NO2 Lewis Structure How to Draw the Lewis Structure for NO2 YouTube
We start with the lewis. Molecular geometry, dipole moment, and electronegativity. It is a paramagnetic, bent molecule with c 2v point group symmetry.
The molecular formula no₂⁺ indicates a positively charged nitrogen dioxide ion.
You have already seen that covalent bonds are polar when they link two different atoms. This is due to the presence of a lone electron on the nitrogen atom, which creates a bent. Polarity in a molecule occurs due to the unequal sharing of valence electrons; The nitronium ion has a symmetrical shape with no net charge difference, resulting in it being a nonpolar molecule.
To determine whether nitrite (no₂⁻) is polar or nonpolar, we can examine it from three key perspectives: Despite having polar bonds, the. Is no2+ polar or nonpolar? Although the difference is quite less, we have an asymmetrical bent molecular structure that induces the net dipole moment and makes no2 a polar molecule in reality.

Is NO2 Polar or Nonpolar? (Nitrogen dioxide) YouTube

Is NO2+ (Nitronium Ion) Polar or Nonpolar? YouTube

NO2 Lewis Structure How to Draw the Lewis Structure for NO2 YouTube
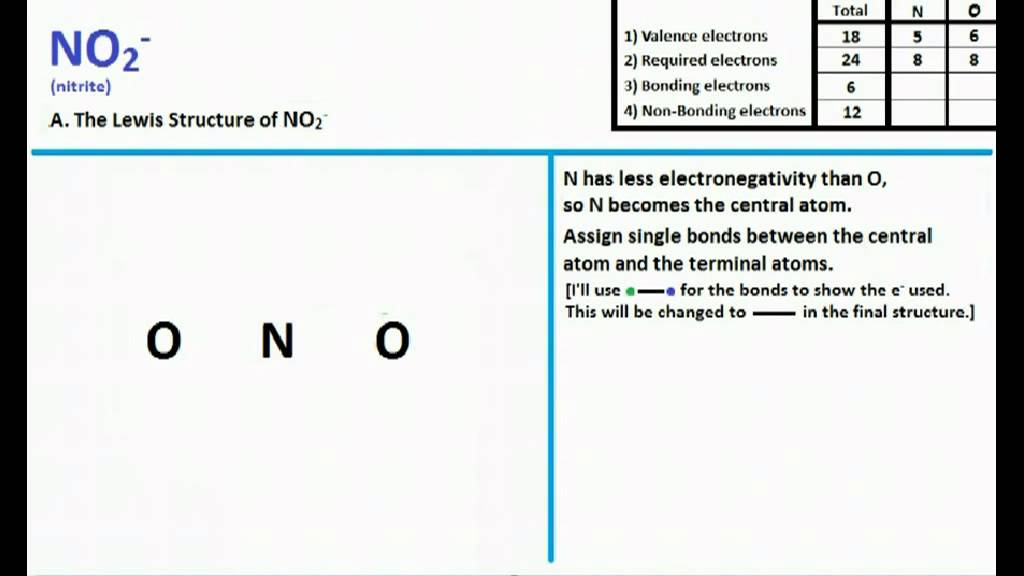
NO2 Lewis Structure and Molecular Geometry YouTube